Mucosal Immunology
The gastrointestinal tract harbours the largest number of immune cells in the body that are separated from our microbial residents by just a single layer of epithelial cells. The mucosal immune system recognizes microbial components through several families of innate immune receptors, resulting in the production of cytokines and antimicrobial proteins that maintain intestinal barrier integrity. Immune cell populations in the GI tract include T, B, innate lymphoid and myeloid cells that act in concert with epithelial and stromal cell populations to mount effector immune responses against invasive pathogens while at the same time avoiding deleterious responses to commensals. This dynamic cross-talk therefore ensures the coexistence of the immune system with the microbiota in a mutually beneficial relationship and is essential to maintain intestinal homeostasis. Perturbation of this delicate equilibrium – through genetic, bacterial or environmental factors – can lead to the breakdown of homeostasis and to the development of chronic inflammatory diseases such as Inflammatory bowel diseases (IBD) and associated cancers.
In our group, we are interested in understanding how myeloid innate cells – and in particular eosinophils, a highly abundant cellular subset residing in the gastrointestinal tract – control the balance between intestinal homeostasis and inflammation, and contribute to the development of colorectal cancer. We are using several models of colitis, bacterial infections and colorectal cancer, together with new tools specifically targeting the eosinophil lineage, to investigate the pathways used by eosinophils to sense activating cytokine and bacterial signals, and the mechanisms through which these activating signals translate into a functional response.
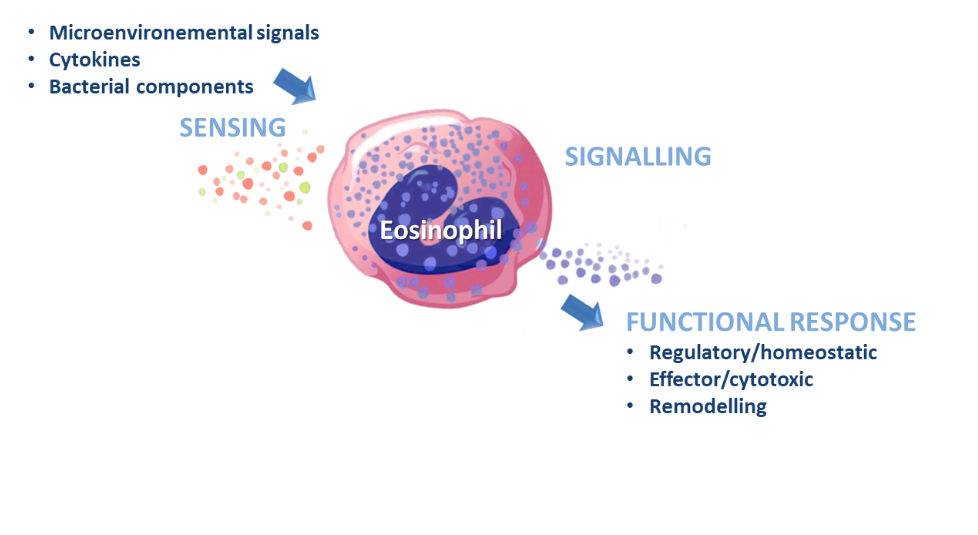
Current research projects focus on intestinal eosinophils and their roles in:
• Regulation of Th1/Th17 immune responses
• Response to commensals and protection against intestinal pathogens
• Promotion of cancer immunity
Recent publications on this topic include:
Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation
Also read comment in: Eosinophils can more than kill
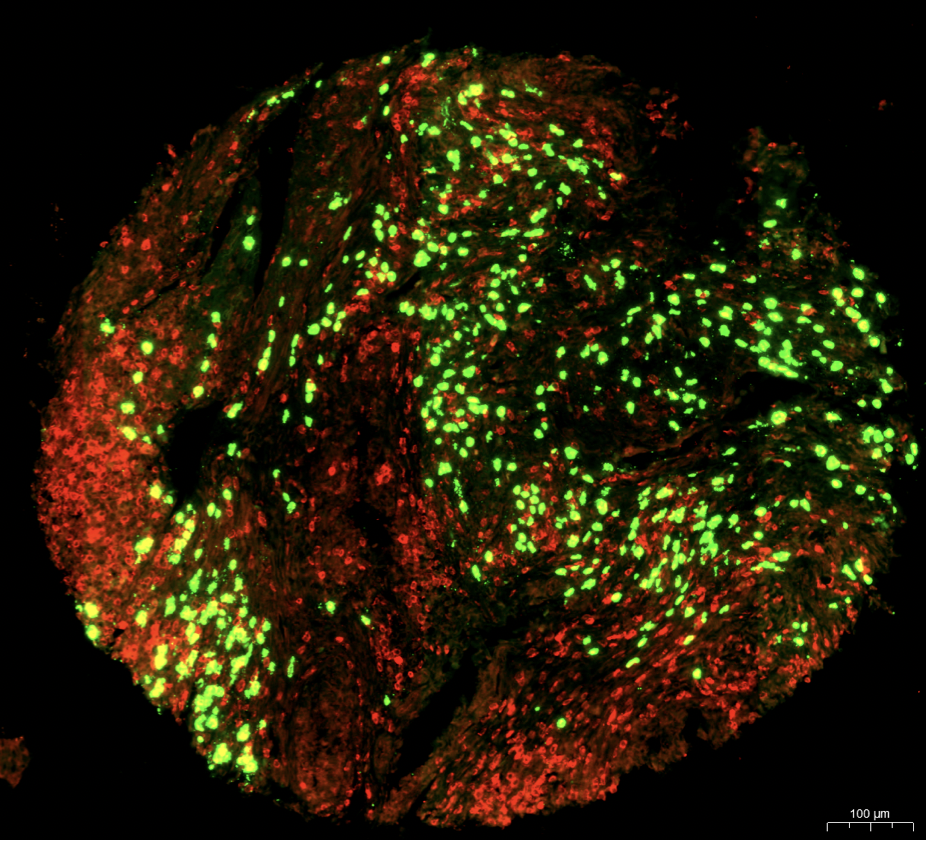
In the steady state gastrointestinal tract, eosinophils account for 20-30% of the total leukocytes and further markedly accumulate in the intestinal lamina propria under inflammatory conditions. However, the contribution of eosinophils to the regulation of local immune responses as well as their role in protective immunity against intestinal pathogens are still poorly understood.
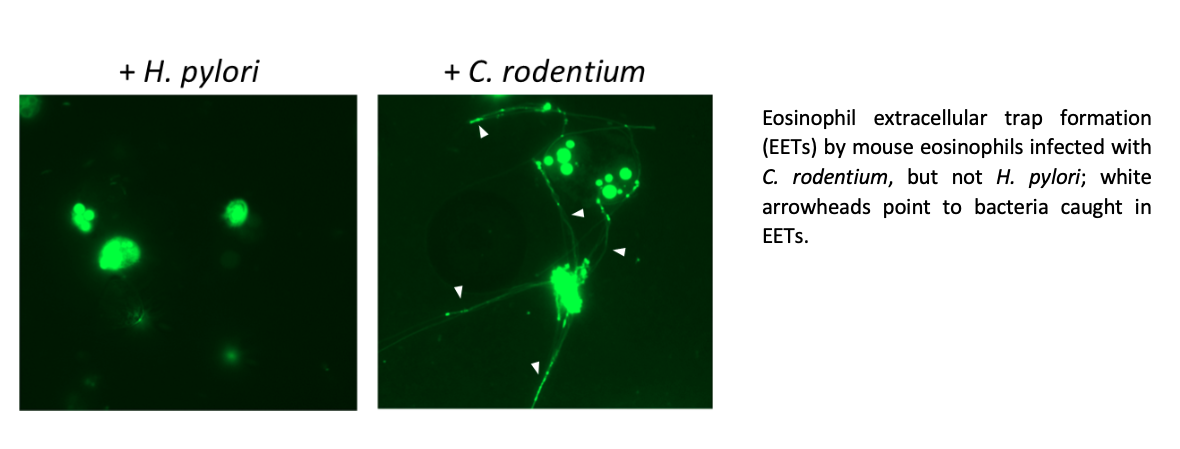
Here we show that eosinophils promote intestinal homeostasis by rather selectively suppressing Th1 and to a lesser extent, Th17 responses against commensals in the colon as well as in models of Helicobacter pylori and Citrobacter rodentium infection. The homeostatic function of eosinophils was regulated by the cytokine IFN-γ, as the ablation of IFN-γ signaling specifically in eosinophils phenocopied the effects of eosinophil depletion at steady state and in all infection models. Eosinophils further possess bactericidal properties that require their degranulation and the deployment of extracellular DNA traps (EETs) against C. rodentium, but not H. pylori. The released DNA forms a localised scaffold that captures both eosinophil granule proteins and bacteria, thereby ensuring the targeted killing of pathogens while limiting cytotoxic damages to the surrounding tissues. This bactericidal activity might be particularly important in situations where the epithelial barrier integrity is compromised such as in IBD, and where EETs might provide a second physical barrier to limit bacterial invasion. However, the bacterial susceptibility to eosinophil killing and EETs is highly variable among different bacterial species and the mechanisms through which some species evade eosinophil recognition still need further investigation.
Also read comment in: Stop Press: Eosinophils Drafted to Join the Th17 Team
The role of intestinal eosinophils in immune homeostasis is enigmatic and the molecular signals that turn them from protective to tissue damaging are unknown. This work was one of the first to demonstrate that the sustained activation of eosinophils through the Th17-derived cytokine GM-CSF promotes pathology during experimental colitis induced by the bacterium H. hepaticus in immunocompromised hosts. In this model, GM-CSF promotes the deregulated expansion of eosinophils in the bone-marrow and acts locally to induce their degranulation, resulting in the chronic release of tissue-toxic eosinophil peroxidase. These findings reveal a new role for eosinophils as key effector cells of IL-23-mediated immune diseases and highlight the GM-CSF-eosinophil axis as an attractive therapeutic target in IBD.
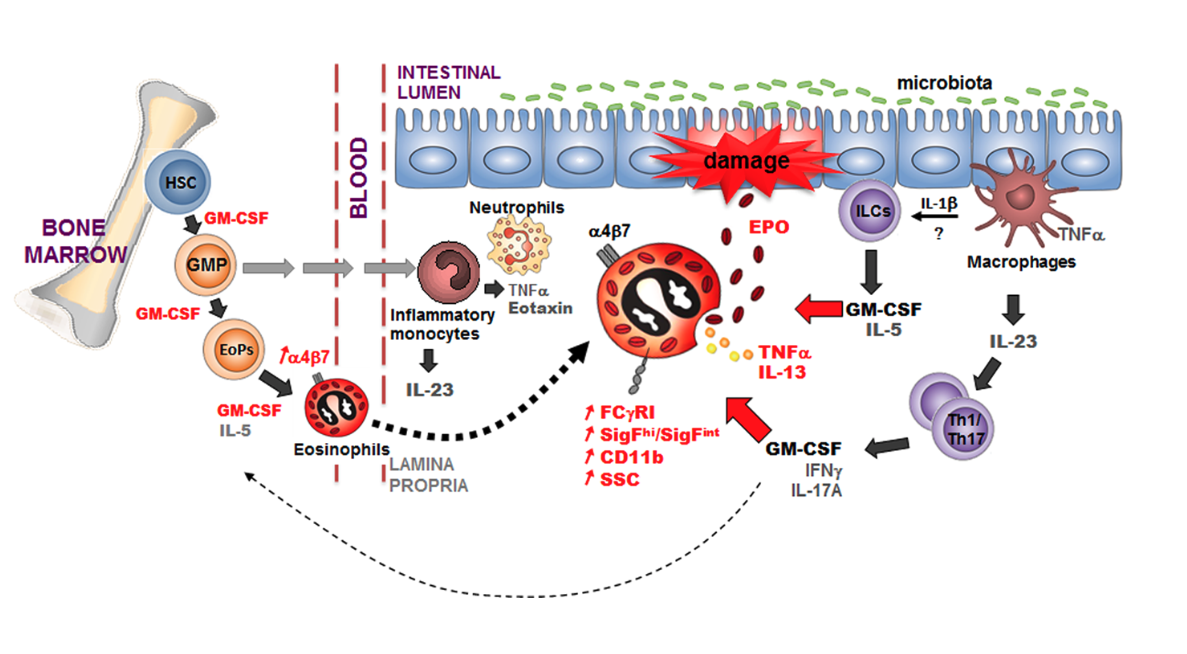
GM-CSF promotes the accumulation and effector functions of tissue-toxic eosinophils in IL-23 driven chronic colitis