Navigation auf uzh.ch
Navigation auf uzh.ch
BACKGROUND
A considerable proportion of tumor tissue consists of recruited and resident cells, often referred to as tumor microenvironment (TME). It is now accepted that the TME influences tumor progression as well as response to standard and immune therapies.
Tumors that are heavily infiltrated by T-cells, especially be CD8+ T-cells, are commonly referred to as “hot” tumors. Such tumors are often characterized by a higher mutational burden and a type-I-interferon signature. Importantly, T-cell-infiltrated tumors respond better to immunotherapy and in fact, the recently developed ImmunoScore – of which the density of T-cells in the tumor is an important parameter – is a frequently used biomarker for prognostic and predictive purposes.
Tumor-associated CD8+ T-cells are very heterogeneous and comprise effector, memory, exhausted as well as the recently described stem-like CD8+ T-cells. Especially the latter subtype seems essential for a clinical response to immune checkpoint inhibition.
AIM
We focus on the mutual interaction between cancer and the immune system with the goal of enhancing tumor-specific immunity in a way that it can be transferred to cancer patients. For this reason, our research questions are inspired by clinical observations, we then address specific aspects in animal models and usually validate these results at least using samples from human cancer patients. This way of working has established our laboratory as an important link between basic and clinical research.
RESEARCH PROJECTS
1. The influence of cancer-cell-intrinsic factors on the TME
The cGAS-STING pathway appears to play a dichotomous role in immunity to cancer: STING-mediated induction of IFN-I provides necessary inflammatory signals but may also negatively affect tumor immune control. We will therefore address questions related to cGAS-STING and the TME.
See also Schadt L, et al. Cell Reports 2019.
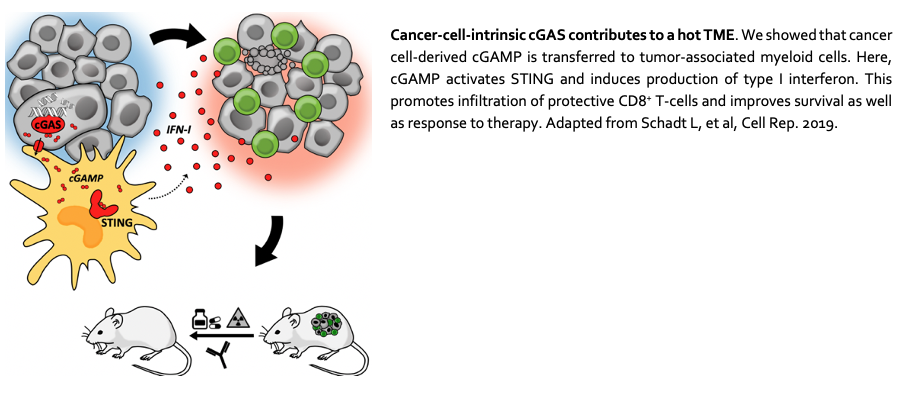
2. Metastatic dormancy: CD8+ T-cell-mediated control of disseminated cancer cells
Metastasis is the major cause of death in patients with breast cancer. Whereas some patients develop metastasis shortly after or even before diagnosis, others show metastatic lesions only years or decades after removal of the primary tumor.
We discovered that dormancy of disseminated cancer cells is controlled by CD8+ T-cells. We will now focus on understanding the processes in the dormant tumor cell niche.
See also Tallón de Lara P, et al. Nature Communications 2021.

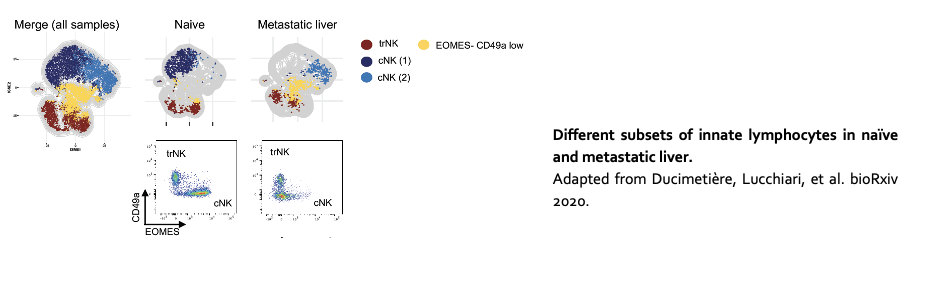
3. Innate immune control of liver metastasis
The liver is one of the most common sites of metastasis. Compared to other organs, the liver harbors a unique immune environment characterized by the abundance of innate cell lineages, including conventional natural killer cells (cNK), natural killer T cells (NKT), and tissue type 1 innate lymphoid cells (trILC1s). We investigate the interplay between these innate lymphoid cells and disseminated cancer cells in the liver.
See also Ducimetière L, Lucchiari G, et al. https://biorxiv.org/cgi/content/short/2020.07.17.206433v1.
4. Tertiary lymphoid structures in cancer
Tertiary lymphoid structures (TLS) are organized structures that resemble secondary lymphoid organ (SLO) follicles in both function and architecture. In most cancer patients, a high density of tumor-associated TLS correlates with a good prognosis. Currently, little is known about the signals and cell types that drive the development of (cancer-associated) TLS. We investigate the sequence of events in TLS development as well as their predictive value in the context of immunotherapy.
See also Silina K, et al. Cancer Research 2017; van Dijk N, et al. Nature Medicine 2020.

METHODS AND TECHNIQUES